Treatment history
Prior to 1995 there were no FDA approved drugs to treat Pulmonary Hypertension. The only way to help a patient before that time was placing them on a transplant list for a Heart and Double lungs.
Today we have a total of 14 FDA approved drugs to treat, NOT CURE, Pulmonary Hypertension. Of those 14 drugs we only have ONE FDA approved medication to treat Pediatric patients from the age of 3 to adult and that is Bosentan.
Medications in the same class as one another CANNOT be used together. You are able to use 3 pathways for the treatment and control of PH, three is the most aggressive process in getting the disease under control.
You can see the average time span from one medication to the next for FDA approval of treatments for PH.

Our first approved medication was Flolan and Veletri, they were approved in 1995. This is an Intravenous medication that uses a pump and hickman line directly to the heart for distribution of medication. Both medications are administered the same way, however; they are different. Flolan has a different additive glycine-mannitol excipients and Veletri arginine-mannitol excipients. Excipient means: Inactive substance to make the workings of the medicine.
6 years between approval
 Bosentan (Tracleer®) is an Endothelin Antagonist Receptor (ERA) oral medication that helps prevent the blood vessels from narrowing. This medication was FDA approved for Patients in 2001 and recently the First FDA approved therapy for Children under the age of 18 for use. There is currently three different milligram dosages for a patient to take. There are a total of three ERA medications, which we will discuss later on. Bosentan was FDA approved in 2001 for the treatment of PH.
Bosentan (Tracleer®) is an Endothelin Antagonist Receptor (ERA) oral medication that helps prevent the blood vessels from narrowing. This medication was FDA approved for Patients in 2001 and recently the First FDA approved therapy for Children under the age of 18 for use. There is currently three different milligram dosages for a patient to take. There are a total of three ERA medications, which we will discuss later on. Bosentan was FDA approved in 2001 for the treatment of PH.1 year between approval

 Treprostinil (RemodulinⓇ) Treprostinil is a synthetic analogue of prostacyclin, a naturally occurring substance in the body, which has effects on dilating blood vessels. Subcutaneous infusion was FDA approved in 2002, picture to the left. Intravenous with a hickman line direct to heart was FDA approved in 2004, picture on the right. This is the same type of intravenous administration as flolan which is pictured above.
Treprostinil (RemodulinⓇ) Treprostinil is a synthetic analogue of prostacyclin, a naturally occurring substance in the body, which has effects on dilating blood vessels. Subcutaneous infusion was FDA approved in 2002, picture to the left. Intravenous with a hickman line direct to heart was FDA approved in 2004, picture on the right. This is the same type of intravenous administration as flolan which is pictured above.
Iloprost (Ventavis®) Iloprost is a synthetic analogue of prostacyclin, a naturally occurring substance in the body, which has effects on dilating blood vessels. FDA approved in 2004. This is like a mini nebulizer which is an inhaled treatment.

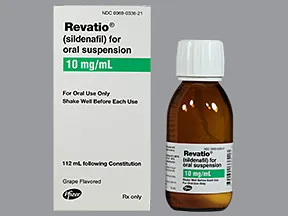 Revatio (Sildenafil™) is a Phosphodiesterase Inhibitor or PDE 5 which helps the lungs produce their own natural vasodilators. This medication was
Revatio (Sildenafil™) is a Phosphodiesterase Inhibitor or PDE 5 which helps the lungs produce their own natural vasodilators. This medication was FDA approved in 2005. There are two forms of this medication Pill form for adults and Liquid for pediatrics. However; it is not FDA approved for children but it can still be administered. There are two PDE5 medications the other will be discussed later.
1 year between approval
2 years between approval

Ambrisentan (LetairisⓇ) is also an ERA like that of Tracleer, that is orally administered. This drug was FDA approved in 2007.
Tadalafil (AdcircaⓇ) Another PDE5 oral medication (one of my therapies) was FDA approved in 2007. The same type of drug as Revatio.
2 years between approval
Treprostinil INHALED (Tyvaso®) is the inhaled version of Remdoulin and was FDA approved in 2009
4 years between approval
Riociguat (Adempas®) Soluble Guanylate Cyclase (sGC) Stimulators increase the interaction of sGC with another chemical (nitric oxide) to help the blood vessels in the lungs relax. FDA approved in 2013.


Oral Treprostinil (Orenitram®) oral version of Remodulin. FDA approved late 2013.
2 years between approval
Selexipag (Uptravi®) Selective IP Receptor Agonist targets and activates a prostacyclin receptor helping the blood vessels in the lungs relax. FDA approved in 2015.

I hope this information helped you to get an understanding of the different types of medication used to help treat, not cure, pulmonary hypertension. Please join me with my discussion of Clinical trials 😊💜






No comments:
Post a Comment